Chapter 5
Atomic Structure and Processes
5.1 Elementary atomic structure
Bohr Orbits correspond to principal quantum number n.
Hydrogen atom energy levels
where the Rydberg energy is
|
Ry = |
me
2
|
| ⎛
⎝
|
e2
4πϵ0ħ
| ⎞
⎠
|
2
|
= 13.6 eV . |
| (5.2) |
The principal quantum number corresponds to the number of nodes of the
radial solution of Schrödinger's equation. In the semi-classical
treatment of Bohr, the energy of a circular orbit with angular
momentum nħ is equal to En, but it is important to realize
that in proper quantum mechanics, there are many possible different
values of angular momentum for any principal quantum number n (from
zero up to approximately nħ.)
The wave function for the hydrogen ground state can be written
where a0 is called the Bohr radius,
|
a0 = | ⎛
⎝
|
ħ
me
| ⎞
⎠
|
| ⎛
⎝
|
4πϵ0
e2
| ⎞
⎠
|
= 5.292×10−11 m . |
| (5.4) |
Angular momentum of the electron orbit is quantized by a quantum number
l. The actual angular momentum is √{l(l+1)} times Planck's
constant ħ, which is approximately lħ for large l. The
quantum number l can take any integer value up to n−1. For the
hydrogen atom the energy levels are (almost) unaffected by l but for
multiple-electron atoms the energy differences between different l
levels become large. In the wave-function, l is the number of the
spherical harmonic (Plm(cosθ)) in its angular
variation. Roughly speaking, l is the number of nodes of the
wave-function.
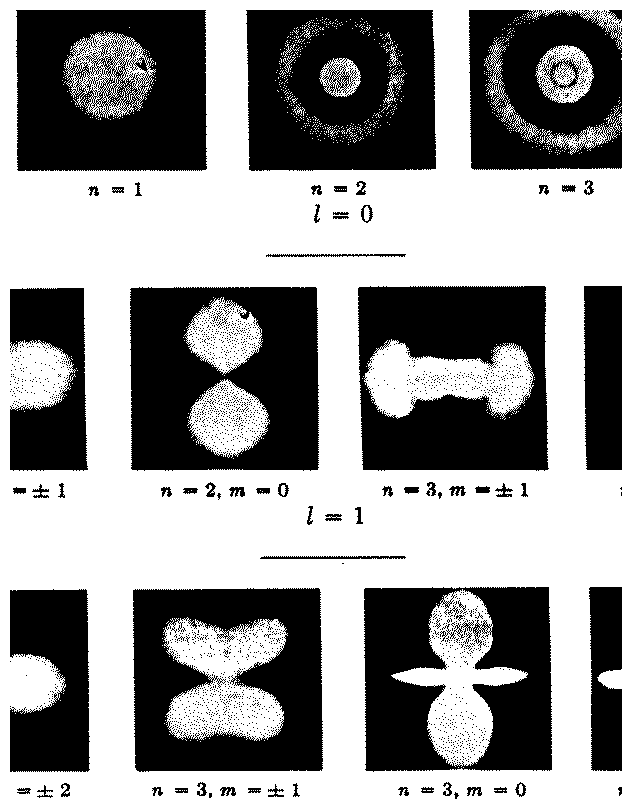
Figure 5.1:
Representation of the electron cloud
surrounding the nucleus for different quantum numbers. [From Herzberg]
5.2 Atomic processes in electromagnetic interactions
Atoms can emit or absorb radiation when their electrons make a
transition from one quantum state to another. If the energies of the
initial and final states are Ei and Ej, then the energy of the
photon emitted (or absorbed) is hνij = |Ei − Ej| and obviously
the conservation of energy means that Ei must be greater than
Ej for emission and less than it for absorption.
Absorption normally takes place by electric dipole transitions. If the
energy density of radiation of frequency ν is ρ(ν) per unit
frequency (ν), per unit volume, then the transition probability per unit
time from the lower to the upper state is written ρ(νij)Bji with the Einstein coefficient, Bji given by Fermi's
golden rule
|
Bji = |
8π3
3h2
|
|
Sij
4πϵ0
|
= |
8π3
3h2
|
|
1
4πϵ0
|
| ⎢
⎢
| ⌠
⌡
|
ψi erψj*d3r | ⎢
⎢
|
2
|
. |
| (5.5) |
The quantity Sij is the square magnitude of the "matrix
element" of the atomic dipole moment. (Single quantum states, are
considered here to avoid worrying about statistical weight.)
Emission of radiation by the atom if the electron is in the upper
state can also be induced by the presence of a radiation field. Its
rate per unit time is ρ(νij)Bij with the emission
coefficient, Bij equal to the absorption coefficient Bji.
Even in the absence of background radiation, "spontaneous" emission
occurs with a probability per unit time of Aij = Bij 8πhνij/c3. A typical order of magnitude for 1/Aij, which is
the lifetime of the excited state, is nanoseconds. As a result, atoms
generally spend most of their time in the ground-state, the state of
lowest energy, where electrons fill up all the low energy states as
far as possible consistent with the exclusion principle.
Selection rules for which transitions are allowed by electric dipole
radiation arise from the fact that the matrix elements, Sij, are
zero unless, for example, ∆l = ±1. The energy levels are
often illustrated graphically using what is called a "Grotrian"
diagram, with energy (often measured in units of cm−1
corresponding to the inverse of the wavelength, 1/λ = E/hc)
indicated by height, and angular momentum quantum
level on the abscissa. Figure 5.2(a) shows the levels for
hydrogen, which has equal energy for different l values. Figure
5.2(b) shows scaled energies for several different
elements but with different levels of ionization so that they all
possess three electrons, making them "lithium-like". The angular
momentum degeneracy is broken, and the active electron can occupy only
the principal level n=2 and above, since the n=1 level is already
filled with the other two electrons.
(a)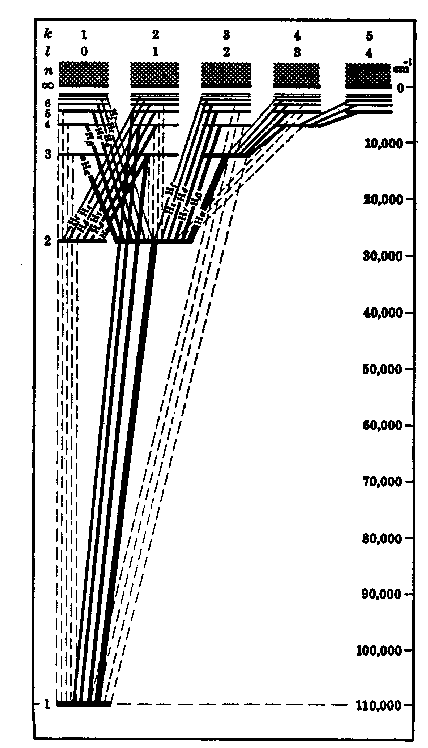 (b)
(b)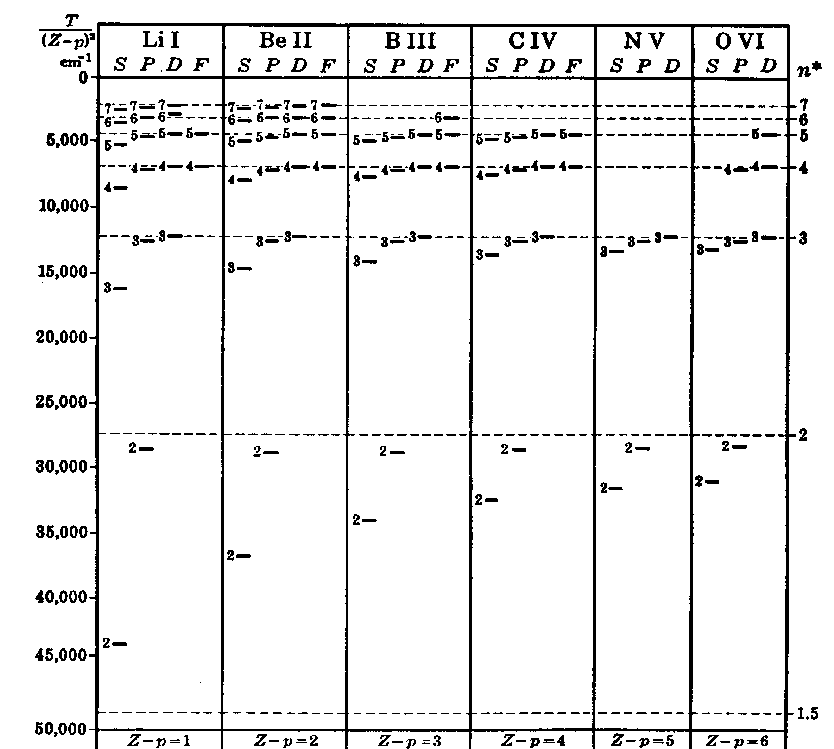 |
Figure 5.2: Energy level
diagrams for (a) Hydrogen, indicating the allowed transitions, especially
the Balmer series, (b) Lithium-like configurations arising from
different stages of ionization, each possessing three electrons.[After
Herzberg.]
When both the upper and lower energy states of the electron are bound
states, the radiation emission occurs as a discrete narrow line at the
specific frequency νij. Line radiation is characteristic of a
particular element and is one of the most powerful means of
identification. However, transitions can also occur between bound
states and free electron states. In the case of a downward transition,
this is a process of "recombination" whereby an initially ionized
atom recombines with a free electron, emitting the excess energy in
the form of an electromagnetic photon, and forming a composite
atom. In that case, because there is a continuous range of possible
kinetic energies for the free electron (unlike the situation with a
bound upper state) there is a continuous spectrum of electromagnetic
radiation from this process. The opposite process is when a photon
excites an initially bound electron into a free state. This is the
process of photo-ionization of an atom, known more colloquially as the
photoelectric effect.
For multiple-electron atoms the electrons are said to reside in
"shells". These shells correspond to the principal quantum
levels. The lowest energy states, corresponding to electrons bound
most strongly to the nucleus, are the n=1 level, known as the
K-shell. The next n=2 level is known as the L-shell, and so on. An
atom of a relatively heavy element has several shells filled with
electrons. For example copper has the K, L, and M-shells filled and
one electron in the N-shell. The easiest electron to remove from the
atom is the electron in the highest energy state. Its binding energy
determines the "ionization energy" of the element, that is, the energy
that needs to be provided to strip the electron off (7.72 eV for
copper). The K-shell electrons are much more strongly bound. Their
binding energy is roughly Z2Ry, (more precisely, for copper 9.0
keV, corresponding to a wavelength of 0.138 nm).
If a photoelectric absorption removes an inner shell
(e.g. K-shell) electron, as is often the case, then the resulting
partially ionized atom is left in an excited state. Moreover, the
excitation energy far exceeds the ionization energy of the resulting
atom. There is, then, a hole in a very deep shell and electrons from
other higher shells can liberate a lot of energy (roughly the K-shell
binding energy) if they make a transition down into the hole. One of
the easiest ways for this transition to occur is for the excess energy
to be given to one of the weakly bound electrons in the highest
shell. Since the energy exceeds the ionization energy, the weakly
bound electron becomes completely unbound and is ejected from the atom
with the excess energy less its binding energy appearing in its
kinetic energy. This process is called the Auger effect (or sometimes
"autoionization") and the ejected electrons are called Auger
electrons.
5.3 The Photoelectric Effect
It is interesting to contrast the photoelectric effect with Compton
scattering. Both of these processes describe a photon interacting with
an electron, transferring energy to it, and ejecting it from its
atom. The key differences are
- In Compton scattering the combined momentum of the electron and
photon is conserved, whereas in photoelectric absorption, momentum is
transferred to the nucleus of the atom. Consequently:
- In Compton scattering, a photon emerges from the
interaction carrying away substantial energy and momentum, whereas the
photoelectric effect involves absorption of the entire photon
energy and its transferral to binding and kinetic energy of the
electron.
- Compton
scattering is important only when the the photon energy is at least comparable
to the electron rest energy, whereas the photoelectric
cross-section increases strongly as the photon energy decreases, and
completely dominates the photon absorption for energies less than
roughly 100 keV.
The cross-section for photoelectric absorption is not straightforward
to calculate rigorously. Some of the earliest calculations, prior to
the full development of quantum mechanics (Kramers 1923), used
classical radiation theory and the "correspondence principle" to
obtain (quite accurate) estimates based on a calculation of the
inverse process, radiative recombination, and hence deducing the
photoelectric cross-section from equilibrium arguments and the
principle of detailed balance. These non-relativistic calculations can
be carried through using the bremsstrahlung formulism we shall discuss
later, but the time is probably not well spent here. The result of
these calculations is to obtain a cross-section for absorption by a
single K-shell electron of photons with energy above the K-shell binding
energy in the form
|
σp = | ⎡
⎣
|
G |
32π2
3√3
|
re2 | ⎤
⎦
|
α3 Z4 | ⎛
⎝
|
mec2
hν
| ⎞
⎠
|
3
|
. |
| (5.6) |
where α is the fine structure constant ( ≈ 1/137), Z
is the nuclear charge, hν = E is the photon energy, and G is a
numerical factor of order unity. Notice that the square bracket factor
is roughly seven times the Thomson cross-section (8πre2/3).
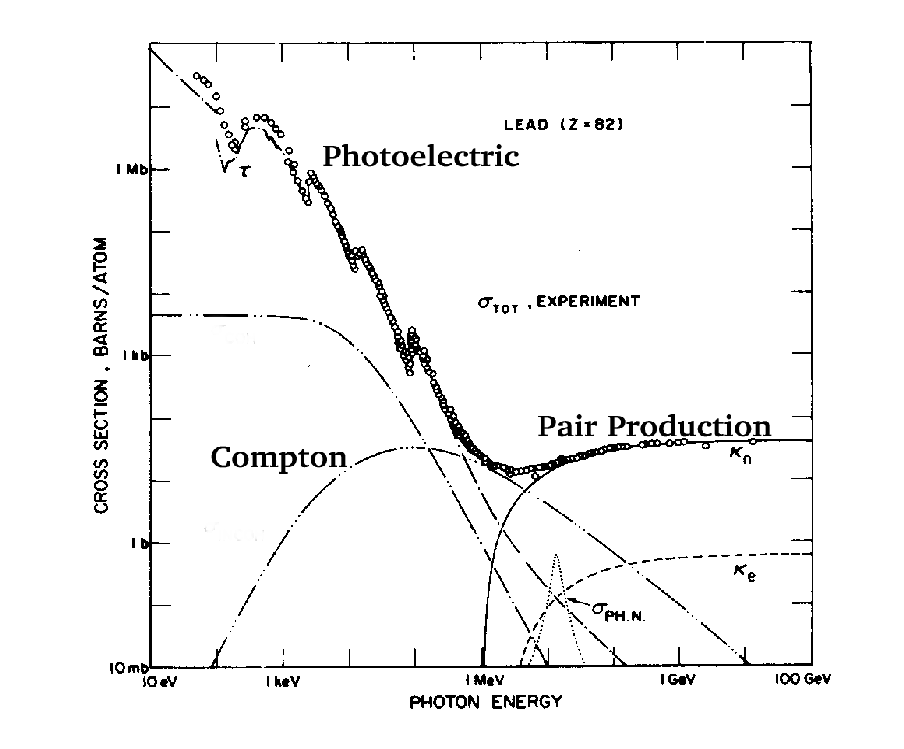
Figure 5.3:
Photon
absorption processes for Lead. At low energy the photoelectric effect
dominates, and the absorption edges for the different shells are
visible. [From H.Anderson, Ed.]
|
σp = 4√2 Z5 α4 | ⎛
⎝
|
mec2
hν
| ⎞
⎠
|
3.5
|
σThomson . |
| (5.7) |
while for the strongly relativistic case, E >> mec2 the formula
|
σp = 1.5 Z5 α4 | ⎛
⎝
|
mec2
hν
| ⎞
⎠
|
σThomson |
| (5.8) |
may be used. [Each of these expressions is the cross-section per
atom].
A comprehensive graphical representation for total photon absorption
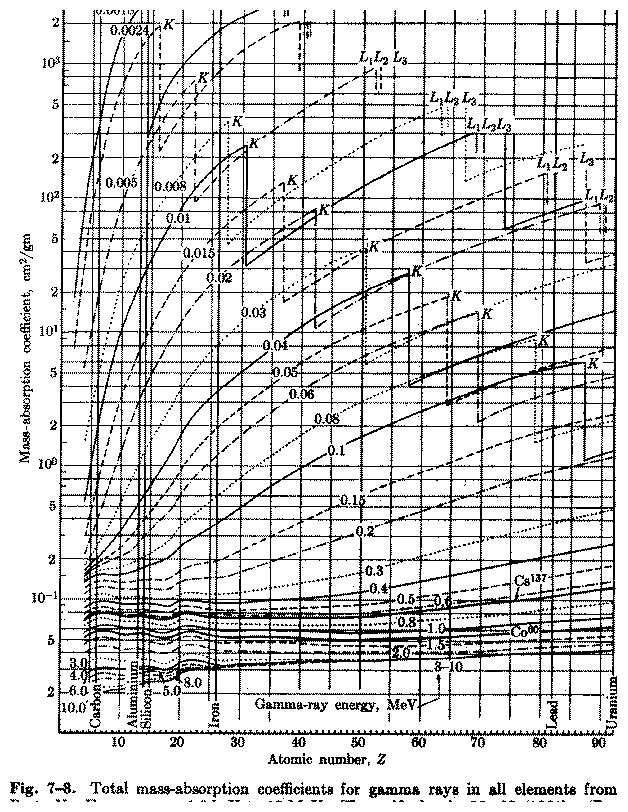
down to 1.8 keV energy is given in Figure 5.4
Figure 5.4:
Absorption coefficients for
all elements. [From Enge]
5.4 Electrons and Pair Production
In 1928 Paul Dirac, by a master-stroke of mathematical insight, developed a
theory of the electron that combines quantum mechanics consistently
with relativity. The theory predicted many hitherto unsuspected
phenomena including especially electron spin, but also that there
exist electron states of negative energy. In order to explain
why electrons do not immediately make a transition from their positive
energy state to a negative energy state, Dirac postulated that all the
negative states are full. In that case, a vacancy or "hole"
in the negative energy states, which naturally has positive
energy with respect to a fully-occupied situation, behaves just like an
electron except that its charge is positive. Thus the positron
was predicted. Actually, Dirac first thought that the holes might
correspond to protons. However a few years later, when the first
experimental evidence of positrons was observed, he immediately saw
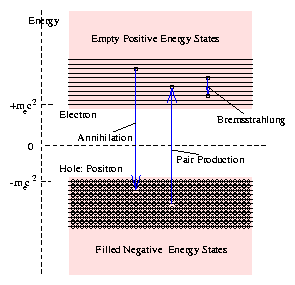
this as confirmation of his theory.
When a positron - a hole in the otherwise full negative energy
states - and an electron encounter one another, a downward
transition of the electron into the lower, negative energy, state can
occur. The electron fills up the hole, annihilating both particles;
its energy becomes negative; and the rest energy (2mec2) and
kinetic energy of the two particles appears as a photon. The analogy
with a electron transition in an atom is helpful, although this
situation really involves two free states of the electron, so it is
more closely allied to bremsstrahlung.
Figure 5.5:
Schematic diagram of positive
and negative energy electron states, annihilation and pair production.
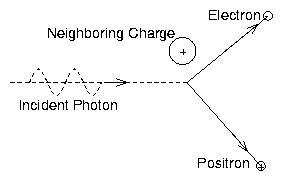
Figure 5.6:
Pair production by an
energetic photon in the presence of a neighboring charge.
|
σpp ≈ Z2 α re2 | ⎛
⎝
|
28
9
|
ln |
2hν
mec2
|
− |
218
27
| ⎞
⎠
|
. |
| (5.9) |
Qualitatively, one can understand these factors as follows. The Z2
factor arises from the strength of the perturbative coupling by a
nucleus of atomic charge Z. The fine structure constant α ≡ e2/4πϵ0ħ c and the classical electron radius re ≡ e2/4πϵ0mec2 are characteristic of coupling between
electromagnetic photons and the electron. The cross-section becomes
essentially constant for very high photon energy
hν >> mec2 Z−1/3 α−1 because of shielding of the
nucleus by its bound electrons. Naturally the cross-section falls to
zero as the photon energy is lowered towards 2mec2 = 1.02 MeV.
These characteristics are
illustrated in Figure 5.3. That figure also
shows the coefficient for pair production in interaction with
electrons. It is negligible for heavy elements since the nucleus is
slightly more than Z2 times as effective as an electron and there
are only Z electrons per nucleus. So the total attenuation due to all
electrons is approximately 1/Z times that of the nuclei.
Table 5.1: Atomic Parameters: Definitions and Values
| Rydberg Energy | Ry | (me/2)(e2 / 4πϵ0ħ)2 | 13.61
| eV |
| Bohr Radius | a0 | ħ2 4πϵ0/e2 me | 5.292×10−11 | m |
| Fine Structure Constant | α | e2/4πϵ0ħ c | 1/137.04 | |
| Classical Electron Radius | re | e2/4πϵ0 me c2 | 2.818×10−15 | m |
| Thomson Cross-Section | σT | 8πre2/3 | 6.652×10−29 | m |
| Relationships | | α2 = 2 Ry/me c2 | | |
| | 2Ry a0 = e2/4πϵ0 = αħ c | | |
| | re = a0 α2 | | |
| | ħ = me c a0 α = me c re /α | | |

 (b)
(b)